Nakivo released Backup & Replication 7.0 introducing new feature like the support for Hyper-V and VMware vSphere 6.5.
With the new supported platform, Nakivo extend its backup solution offer allowing the protection of vSphere and EC2 and now also Microsoft Hyper-V environments.
What's new
Support for Hyper-V
Added the support for Microsoft Hyper-V 2016 and 2012. The Hyper-V capability uses the same easy and clean web interface that drives the user during the backup configuration.
The new release provides image-based, application-aware, and forever-incremental Hyper-V backup. Backups are automatically compressed and deduplicated to save space in the backup repositories and backup copies can be sent offsite to AWS and Azure clouds.
You can instantly recover files, Exchange objects, and Active Directory objects directly from compressed and deduplicated Hyper-V backups.
Additional features:
- Agentless, image-based backup of live Hyper-V VMs
- Up to 1,000 GFS recovery points per VM backup
- Full VM recovery to the same or a new host
- Automated backup verification
- Support for Hyper-V Cluster Shared Volumes
Support for vSphere 6.5
Backup & Replication 7.0 now supports the latest VMware vSphere 6.5 release. The new version can back up, replicate, and recover VMware vSphere v6.5 VMs without any extra setup.
Active Directory integration
Nakivo now support Microsoft Active Directory integration to better manage the access to the backup infrastructure following the company’s security policy.
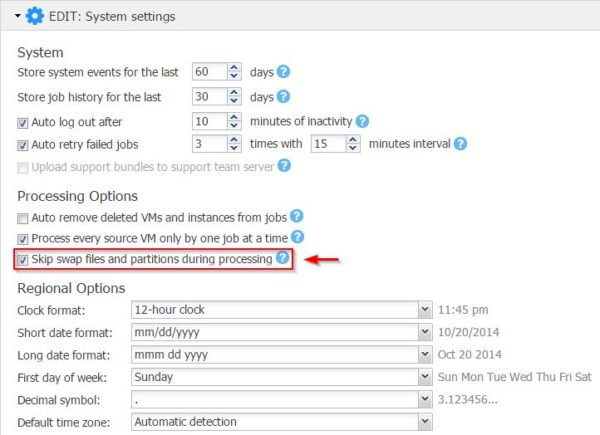
Skip Swap File
The backup of a VM included so far also the swap file since it changes constantly consuming lot of space in the backup repository and making the backup time longer. Version 7.0 provides the capability to automatically skip the swap file in VMs speeding up data transfer and saving space in the backup repository.
Bulk backup delete
Nakivo is now able to delete backups that you no longer need in a easier way. You can filter outdated and orphaned backups and delete them in a single click.
Nakivo Backup & Replication 7.0 is available as 30-day trial for testing purposes.